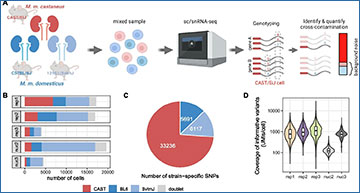
In droplet-based single-cell and single-nucleus RNA-seq experiments, not all reads associated with one cell barcode originate from the encapsulated cell. Such background noise is attributed to spillage from cell-free ambient RNA or barcode swapping events.
Researchers at Ludwig-Maximilians University characterize this background noise exemplified by three scRNA-seq and two snRNA-seq replicates of mouse kidneys. For each experiment, cells from two mouse subspecies are pooled, allowing to identify cross-genotype contaminating molecules and thus profile background noise. Background noise is highly variable across replicates and cells, making up on average 3-35% of the total counts (UMIs) per cell and the researchers find that noise levels are directly proportional to the specificity and detectability of marker genes. In search of the source of background noise, the researchers find multiple lines of evidence that the majority of background molecules originates from ambient RNA. Finally, they use their genotype-based estimates to evaluate the performance of three methods (CellBender, DecontX, SoupX) that are designed to quantify and remove background noise. They find that CellBender provides the most precise estimates of background noise levels and also yields the highest improvement for marker gene detection. By contrast, clustering and classification of cells are fairly robust towards background noise and only small improvements can be achieved by background removal that may come at the cost of distortions in fine structure.
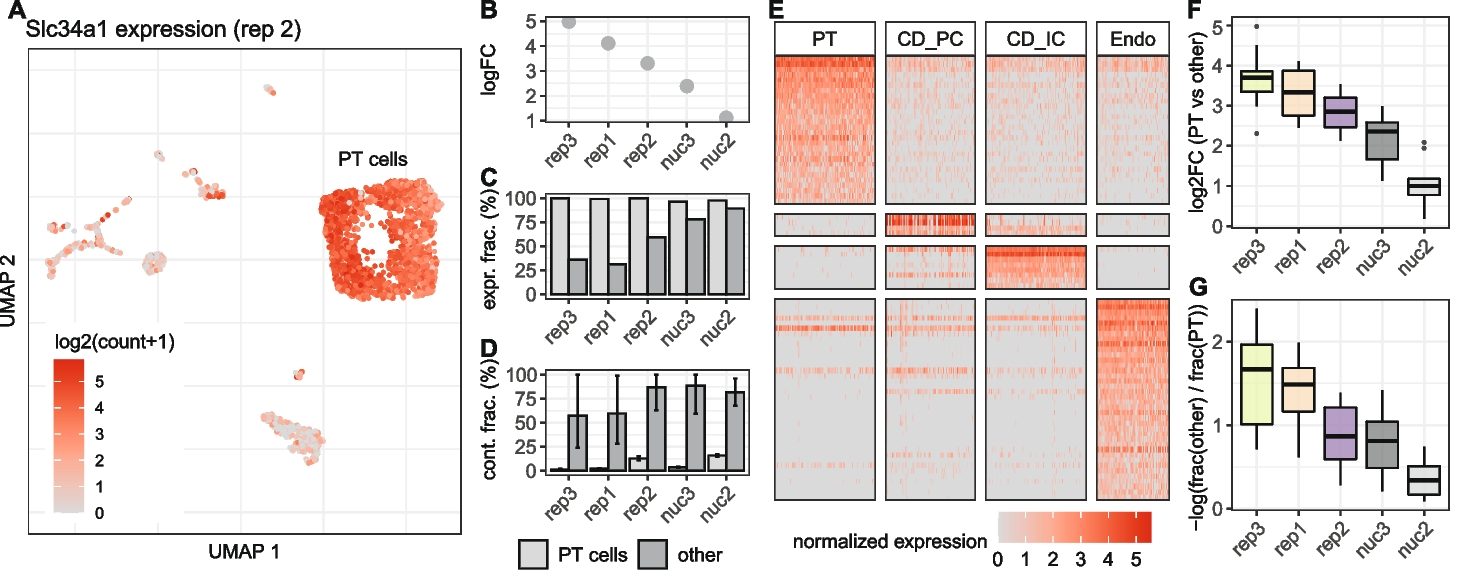
Background noise affects differential expression and specificity of
cell type specific marker genes
A UMAP representation of replicate 2 colored by the expression of Slc34a1, a marker gene for cells of the proximal tubule (PT). Besides high counts in a cluster of PT cells, Slc34a1 is also detected in other cell type clusters. Differential expression analysis between PT and all other cells shows a decrease of the detected log fold change of Slc34a1 (B) at higher background noise levels, as well as an increase of the fraction of non PT cells in which UMI counts of Slc34a1 were detected (C). D Estimation of the background noise fraction of Slc34a1 expression indicates that the majority of counts in non PT cells originates from background noise. Error bars indicate 90% profile likelihood confidence intervals. E Heatmap of marker gene expression for four cell types in replicate 2, downsampled to a maximum of 100 cells per cell type. F Comparison across replicates of log2 fold changes of 10 PT marker genes calculated based on the mean expression in PT cells against mean expression in all other cells. G For the same set of genes as in E, the log ratio of fraction of cells in which a gene was detected in others and PT cells shows how specific the gene is for PT cells
These findings help to better understand the extent, sources and impact of background noise in single-cell experiments and provide guidance on how to deal with it.
Janssen P, Kliesmete Z, Vieth B, Adiconis X, Simmons S, Marshall J, McCabe C, Heyn H, Levin JZ, Enard W, Hellmann I. (2023) The effect of background noise and its removal on the analysis of single-cell expression data. Genome Biol 24(1):140. [article]