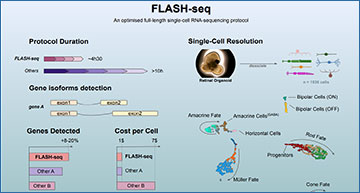
A new single-cell RNA-sequencing protocol developed at IOB enables the detection of a significantly higher number of genes per cell than any existing method. It is also faster, less expensive and more sensitive. The new method has now been published in Nature Biotechnology.
Single-cell RNA-sequencing (scRNA-seq) shows which genes are turned on in a cell and what their level of transcription is. This allows in-depth assessment of the biology of individual cells, and detection of changes that may indicate disease. scRNA-seq is becoming widely used across disciplines including developmental biology, neurology, oncology, immunology, cardiovascular research and infectious diseases. It is critical for studying population heterogeneity, identifying minority sub-populations of interest, and for discovering unique characteristics of individual cells. Applications reach far beyond ophthalmology: single-cell sequencing is widely used for the analysis of de novo germline mutations and somatic mutations in normal and diseased cells, e.g. in cancer cells.
A new scRNA-seq protocol developed at IOB, with scientists from the Novartis Institutes for BioMedical Research, can be used to study any disease model requiring the analysis of rare cell populations at high resolution.
Simone Picelli, Head of the IOB Single-Cell Genomics Platform and senior author of the paper, explains: “Our modular FLASH-seq protocol provides a snapshot of the cell transcriptome at an unprecedented resolution. The method can be miniaturised, automated and adapted to different needs. It helps to define which gene isoforms are present in health and disease. It also provides a much deeper picture of the gene expression, especially after perturbation due to disease, developmental defects or external agents. Moreover, it is easy to set up in the lab, 50% faster and cheaper than similar existing protocols and enables the study of molecular mechanisms of disease beyond the scope of current single-cell sequencing tools.”
FLASH-seq workflow
Steps automated with a liquid handling robot or nanodispenser are marked by a purple and teal square, respectively. Steps/reagents differing from Smart-seq2 are highlighted in red. Step 1, cells are individually sorted in plates containing a lysis buffer. Step 2, after denaturation, mRNAs are reverse transcribed using a template-switching reverse transcriptase (RT) in the presence of an excess of dCTP. Step 3, cDNA is amplified by semi-suppressive PCR. Only a single master mix is required to perform Step 2 and 3. Step 4, cDNA is purified using magnetic beads. Step 5, cDNA concentrations and fragment sizes are measured and the samples are diluted to a final concentration of ~100-200 pg/µl. Step 6, cDNA is tagmented using a Tn5 transposase which introduces known adaptor sequences at both ends of each fragment. Step 7, tagmented cDNA is amplified by PCR and barcoded sequencing adaptors are added. Step 8, samples are pooled. Step 9, the library is purified using magnetic beads. Step 10, concentrations and average fragment sizes are measured in preparation for the sequencing. Steps 4 and 5 are not performed in FS low amplification.
The new method can generate sequencing-ready libraries in just half a day. Researchers at IOB therefore believe that FLASH-seq has the potential to become the tool of choice when looking for an efficient, robust, modular, affordable and automation-friendly full-length scRNA-seq protocol.
Source – Globe Newswire
Hahaut V, Pavlinic D, Carbone W et al. (2022) Fast and highly sensitive full-length single-cell RNA sequencing using FLASH-seq. Nat Biotechnol [Epub ahead of print]. [article]