Late prenatal development of the human neocortex encompasses a critical period of cortical expansion and glial development (cells that are essential to the central nervous system), which is poorly understood, in part due to limited availability of tissue samples from this period. In this study, a research team from the Icahn School of Medicine at Mount Sinai performed high-throughput computational analysis to resolve, for the first time, the entire human glial lineage trajectory during the second and third trimesters of brain development.
The Mount Sinai researchers used single cell transcriptomic analysis of cell type diversity and lineage choice, followed by validation in primary human autopsy tissue to uniquely capture the transience, diversity and lineage choice of cells as the brain grows in middle and late prenatal development, including novel glial progenitor signatures with relevance to disease.
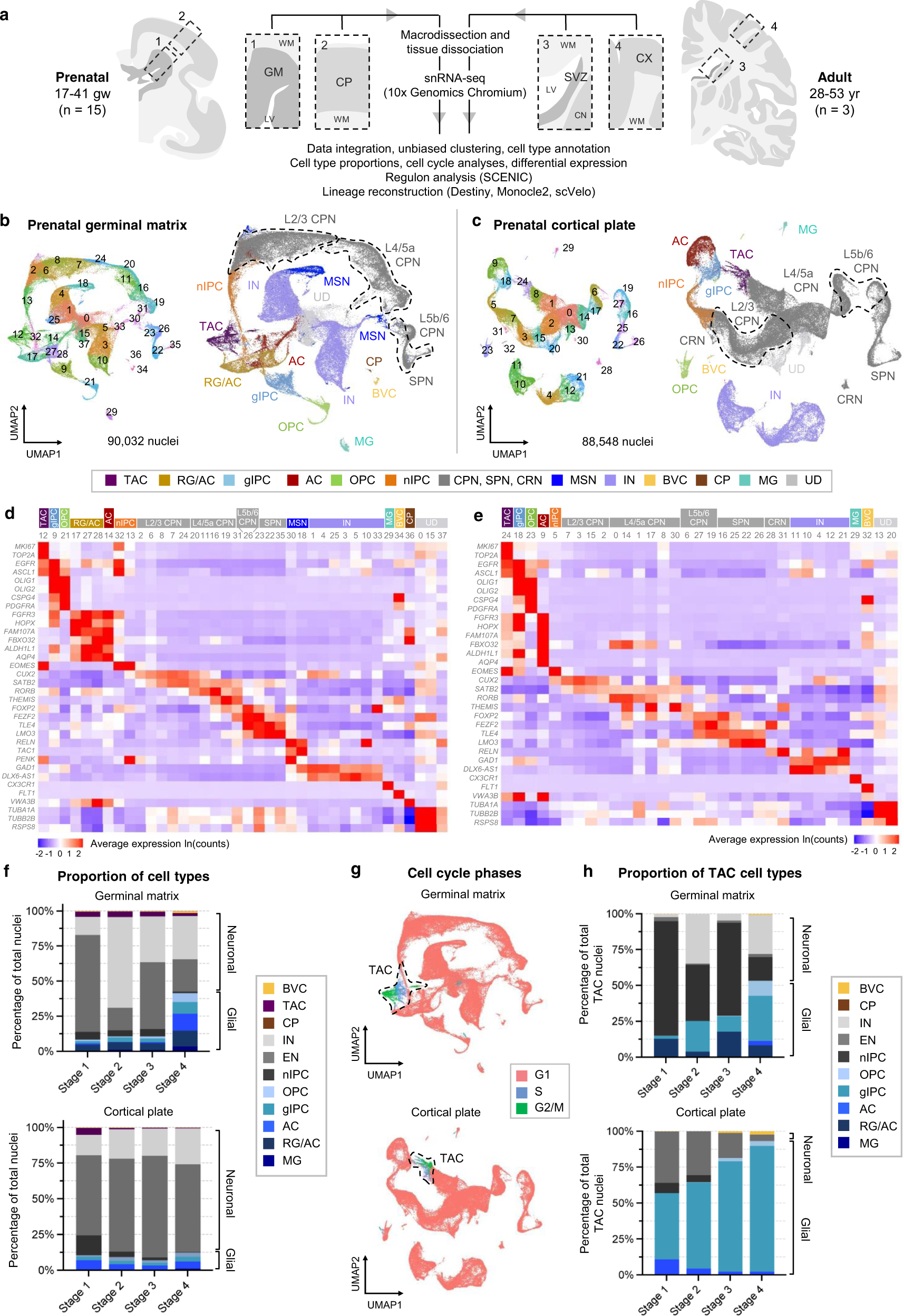
Single nucleus transcriptomic atlas of prenatal and adult human brain regions
a Schematic of macrodissection and sequencing approach to study germinal matrix b, c UMAP plots for all prenatal samples integrated by anatomical region: GM (b) and CP (c). Clusters (left) are colored by cell type annotation (right). d, e Heatmaps of log-normalized average gene expression per cluster, showing selected canonical marker genes (rows) used to assign cell identity to each cluster (columns) in (b) and (c), respectively. Clusters are grouped and colored by cell identity. f Stacked bar plots of stage-normalized cell type proportions in the GM (top) and CP (bottom), showing changes in the factions of glia and neurons over four defined stages of prenatal development: stage 1 (17–20 gw), stage 2 (20–24 gw), stage 3 (24–28 gw), stage 4 (31–41gw). g UMAP display of cell cycle phase annotations within the integrated GM (top) and CP (bottom), highlighting actively cycling cells as TAC. h Stacked bar plot of TAC state cell type proportions in GM (top) and CP (bottom).
“Late prenatal development of the human neocortex encompasses a critical period of cortical expansion and glial development, which is poorly understood, in part due to limited availability of tissue samples from this period. Working together, our team of pathologists, neurodevelopmental biologists, and computational biologists generated a high-throughput atlas of late prenatal human brain development, examining both the niche where stem cells are born and the niche where maturing progenitors migrate to. This atlas uniquely captures both the transience and the diversity of glial and neuronal progenitors during late prenatal growth. Importantly, our study resolves a specific glial progenitor population, gIPC, common to both oligodendrocyte and astrocyte lineages. We also demonstrate the enrichment of newly discovered prenatal glial signatures in several disease states, including intellectual disability, autism spectrum disorder, malformation of cortical development, and gliomas. Beyond this study, our resource dataset motivates further interrogation into both normal human development and clinical association to disease,” said Mount Sinai’s Dr. Tsankova.
Computational analysis uncovers a greater complexity of glial progenitors, including transient glial intermediate progenitor cell (gIPC) and nascent astrocyte populations in the third trimester of human gestation. The research team demonstrate enrichment of specific prenatal glial cell-type signatures in several disease states, including intellectual disability, autism spectrum disorder, malformation of cortical development, and gliomas.
Source – news wise
Ramos SI, Mussa ZM, Falk EN, Pai B, Giotti B, Allette K, Cai P, Dekio F, Sebra R, Beaumont KG, Tsankov AM, Tsankova NM. (2022) An atlas of late prenatal human neurodevelopment resolved by single-nucleus transcriptomics. Nat Commun 13(1):7671. [article]