Functional bone marrow studies have focused primarily on hematopoietic progenitors, leaving limited knowledge about other fragile populations, such as bone marrow adipocytes (BMAds) and megakaryocytes. The isolation of these cells is challenging due to rupture susceptibility and large size. EPFL researchers have developed a label-free cytometry microsystem, MarrowCellDLD, based on deterministic lateral displacement. MarrowCellDLD enables the isolation of large, fragile BM-derived cells based on intrinsic size properties while preserving their viability and functionality. Bone marrow adipocytes, obtained from mouse and human stromal line differentiation, as well as megakaryocytes, from primary human CD34+ hematopoietic stem and progenitor cells, were used for validation. Precise micrometer-range separation cutoffs were adapted for each cell type. Cells were sorted directly in culture media, without pre-labeling steps, and with real-time imaging for quality control. At least 106 cells were retrieved intact per sorting round. This method outperformed two FACS instruments in purity and yield, particularly for large cell size fractions. MarrowCellDLD represents a non-destructive sorting tool for large, fragile BM-derived cells, facilitating the separation of pure populations of BMAds and megakaryocytes to further investigate their physiological and pathological roles.
MarrowCellDLD device and operation
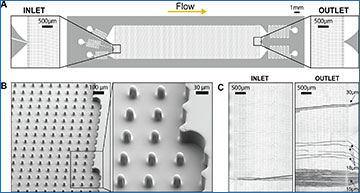
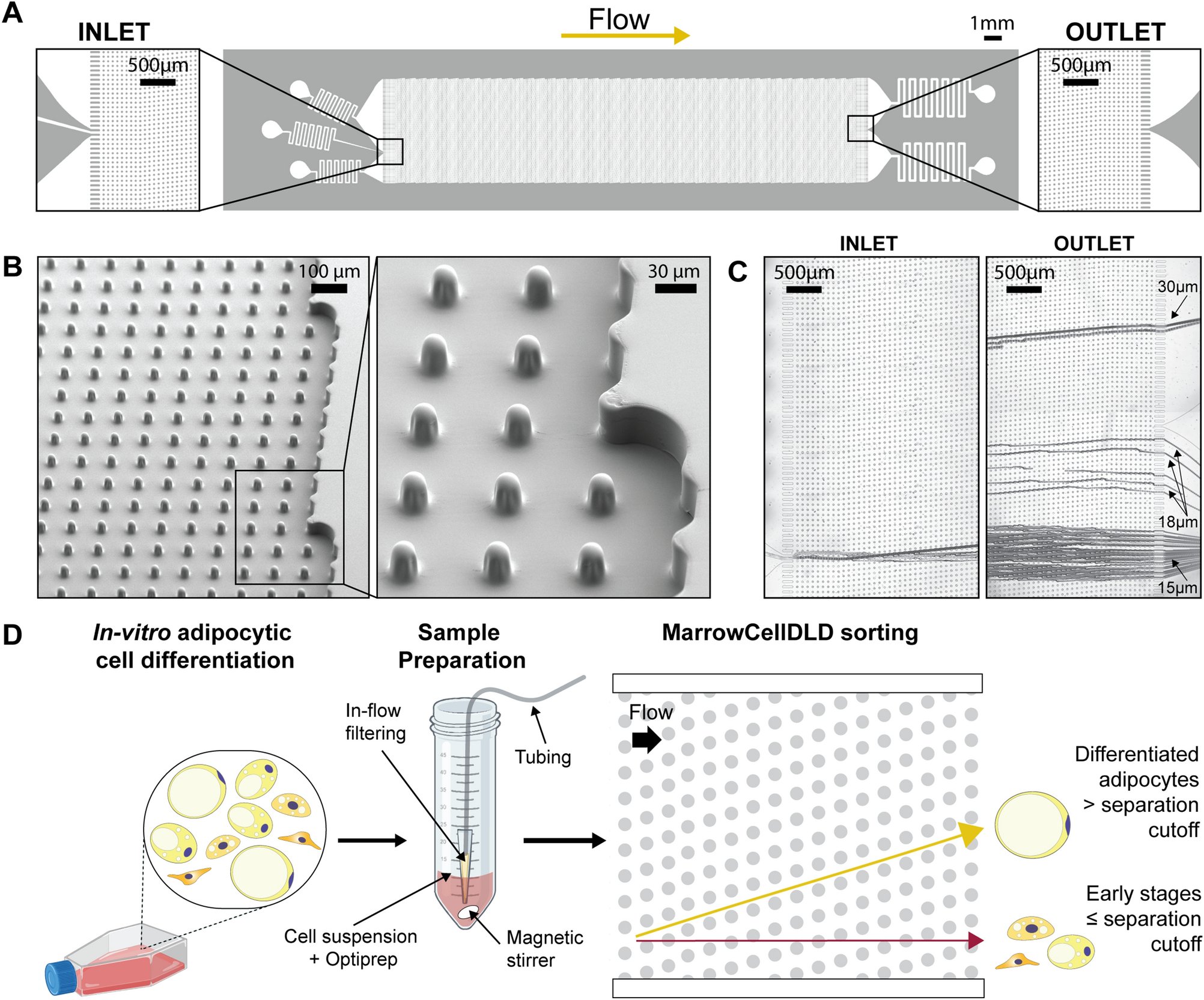
(A) MarrowCellDLD chip: insights on inlet and outlet regions terminated with 10 rows of straight pillars respectively before and after the MarrowCellDLD array active region (tilted array). (B) Scanning electron microscopy images of the MarrowCellDLD array chip with a 19 μm separation cutoff (critical size). (C) Inlet and outlet trajectories of polystyrene microbeads of 15, 18, and 20 μm in size transiting a MarrowCellDLD chip with 19 μm critical size. (D) Experimental workflow to sort differentiated adipocytes by MarrowCellDLD. After adipocytic differentiation in vitro, the induced-OP9 sample contains a mixture of progenitor cells, early stages of differentiation, and mature adipocytes. After trypsinization, the cellular sample is suspended in its original culture media supplemented with Optiprep, then placed at the inlet reservoir connected to the tubing responsible for injecting the sample into the MarrowCellDLD device. A custom in-flow filtering system embedded in the tubing ensures the injection of a single-cell suspension, and the sample is continuously stirred to achieve homogeneity. Within the sorting module, mature adipocytes larger than the critical size for separation should be forced to follow the array angle (displacement mode), allowing for their isolation. Conversely, early stages of differentiation and progenitors should move parallel to the flow (zig–zag mode). The two fractions are thus predicted to be physically separated and can be collected at distinct outlets.
Porro G, Sarkis R, Obergozo C, Godot L, Amato F, Humbert M, Naveiras O, Guiducci C. (2023) MarrowCellDLD: a microfluidic method for label-free retrieval of fragile bone marrow-derived cells. Sci Rep 13(1):22462. [article]