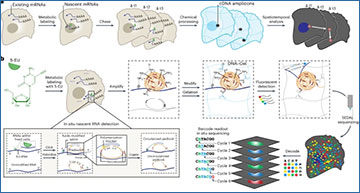
TEMPOmap can follow thousands of newly made RNA molecules within a cell simultaneously, revealing new insight into how genes are controlled.

Credit: Images courtesy of Jingyi Ren; Produced by Scott Sassone, Broad Communications. TEMPOmap identifies RNA molecules as they move within a cell, shown at 1, 2, 3, 4, and 5 hours after they are synthesized.
RNA plays a central role in biology, but there is still much to learn about the molecule’s life cycle in the cell. In recent years, scientists have devised methods that can take snapshots of messenger RNAs (mRNAs) scattered across a cell and measure the ebb and flow of their abundance, but they haven’t yet been able to track the individual movements of a large number of them over time.
A new method built by scientists at the Broad Institute of MIT and Harvard is the first large-scale approach to track mRNAs over both space and time in individual cells. Known as TEMPOmap (temporally resolved in situ sequencing and mapping), the method simultaneously measures the subcellular movements of many RNA molecules, with the potential to follow a molecule from its birth through to its death.
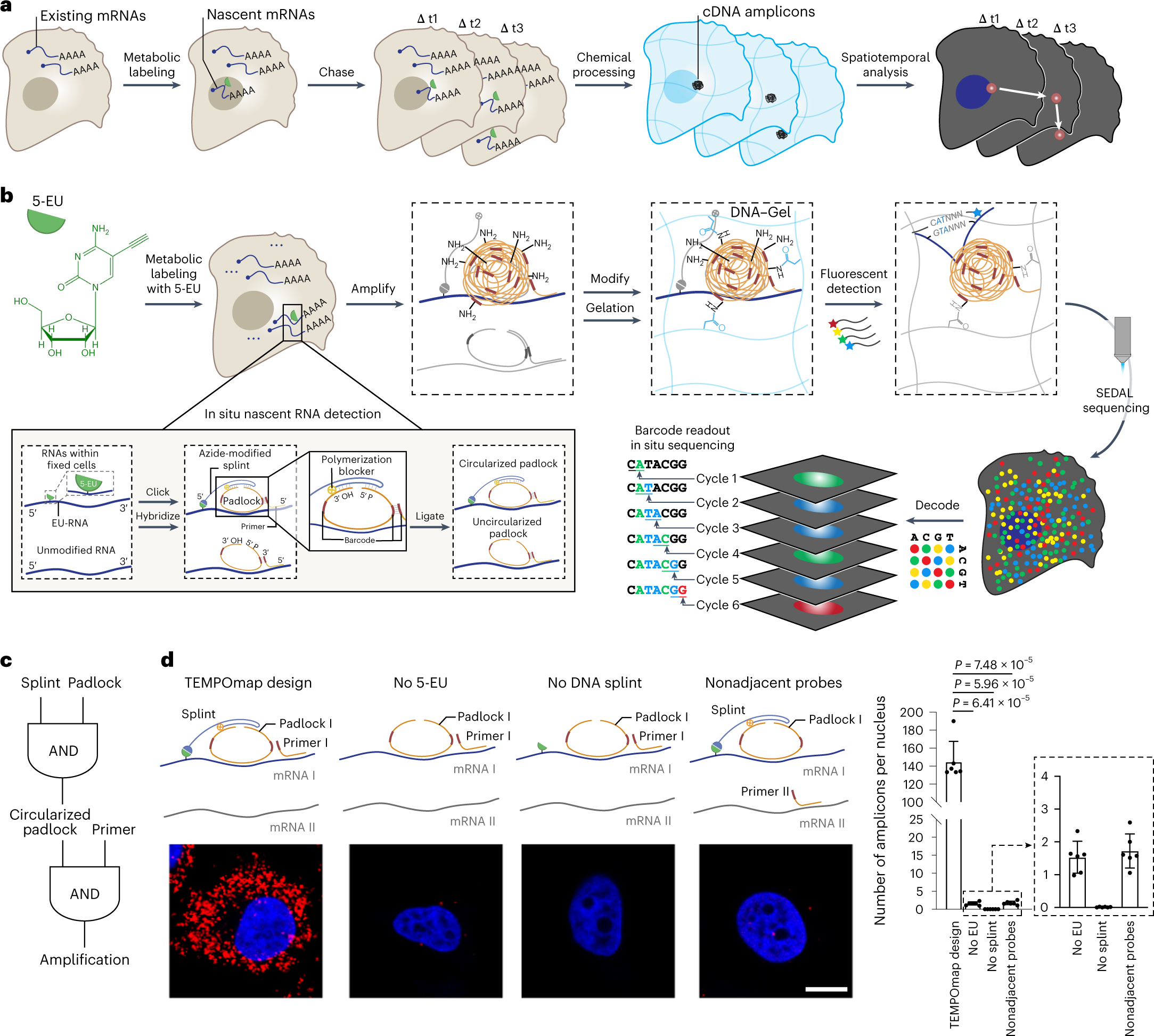
TEMPOmap enables spatiotemporally resolved transcriptomics
a, Overview of TEMPOmap pipeline: nascent RNAs of several timepoints are collected and in situ sequenced, followed by spatiotemporal RNA analyses. b, TEMPOmap experimental workflow. After 5-EU-labeled cells are prepared, a set of tri-probes (splint, primer and padlock) are conjugated or hybridized to cellular mRNAs, resulting in the enzymatic replication of each padlock sequence into cDNA amplicons. The amplicons are anchored in situ via a functionalized acrylic group (blue) to a hydrogel mesh to create a DNA-gel hybrid (blue wavy lines). The five-base barcode on each amplicon is read out by six rounds of SEDAL. Thus, multiplexed RNA quantification reveals gene expression in nascent subcellular locations. c, DNA tri-probe design rationale. The generation of an amplicon requires the presence of splint, circularized padlock and primer probes in proximity. d, Left, schematics and representative fluorescent cell images of negative control experiments of c, showing three-part probe requirement for signal amplification. mRNA_I represents ACTB and mRNA_II represents GAPDH. All four images show ACTB (red) mRNA in HeLa cells (DAPI in blue). Right, quantification of cell images showing the average amplicon reads per cell (n = 6 images were measured containing 310, 585, 386 and 421 cells for each condition from left to right, respectively). Two-tailed t-test. Data shown as mean ± s.d. Scale bar, 10 µm.
TEMPOmap chemically labels newly made RNA molecules and uses a unique approach to sequence them at specific locations in the cell at several time points, allowing researchers to track thousands of RNA molecules in parallel over time. In a study in Nature Methods, the research team described TEMPOmap and how it enabled them to follow mRNAs in a variety of cell types, measure the speed at which the molecules were transcribed from DNA and move within the cell, and discover surprising movements of the RNAs in the cells.
The scientists said maps generated by the approach can help uncover new principles of gene regulation and explore how RNA life cycle patterns influence the function of various cell types in health and disease.
“New ways of measuring the patterns of RNA movement and stability can provide fresh insights into basic principles of molecular biology, in addition to helping us better understand how RNA kinetics impact the function of cells across many different human cell types,” said senior study author Xiao Wang, who is a core institute member and Merkin Institute Fellow at the Broad and an assistant professor of chemistry at MIT.
Time will tell
TEMPOmap builds upon STARmap, a method previously developed by Wang and colleagues that profiles the location of RNA molecules within a cell at one point in time. With TEMPOmap, the Wang lab devised an approach that tags only newly made RNAs as they are being transcribed from DNA, anchors them in place by fixing the cells in a hydrogel, and detects the RNAs at those locations in the cell. By doing so at different time points, the researchers infer the movement of RNA molecules through the cell over several hours.
To detect and sequence the RNAs in individual cells, the team first designed an inventive three-part chemical probe set that precisely binds to the label on a newly made mRNA, forming a circular structure that generates a tight ball of DNA fragments, also known as a “DNA amplicon,” copied from the bound mRNA. The scientists then use an in situ sequencing method known as “SEDAL,” which was devised for the STARmap approach, to analyze the DNA fragments and uncover the identity of the mRNA that’s present at each spot.
The researchers demonstrated the method’s potential by following the subcellular movements of mRNAs representing nearly 1,000 genes in cells in the lab. They also followed mRNAs for several dozen genes in fibroblasts and cardiomyocytes created from reprogrammed human stem cells, and more than 250 genes in human skin cells.
The analysis revealed that mRNAs for genes with a specific function in a specific cell type tend to be synthesized faster and remain more stable in that cell type than in others.
“With TEMPOmap, we’re able to see evidence for these transcripts having a highly functional role in particular cell types,” said co-first author Jingyi (Rena) Ren, a graduate student at MIT and in the Wang lab.
The team’s measurements also revealed the speed of each RNA’s transcription, export from the nucleus, movement through the cytoplasm, and decay. They found that mRNAs displayed different kinetic patterns related to the molecular functions of the genes they encode.
In addition to uncovering new insights into gene expression and control, TEMPOmap can assist studies of other biological phenomena, such as embryonic development, biological clocks, and disease progression. The Wang lab aims to use spatial transcriptomic tools to better understand brain function and dysfunction at the molecular level. For example, they plan to use TEMPOmap to study how gene transcripts travel within brain cells under certain conditions, for example, after a neuron fires.
“Biology is, by nature, a dynamic process,” said Ren. “By following these RNAs, we can help answer some fascinating biological questions.”
Source – The Broad Institute
Availability – The TEMPOmap analysis tool will be maintained and updated at https://github.com/wanglab-broad/TEMPOmap. RNA vector field is generated by Dynamo v.1.0.0 and is available at https://github.com/aristoteleo/dynamo-release.
Ren J, Zhou H, Zeng H, Wang CK, Huang J, Qiu X, Sui X, Li Q, Wu X, Lin Z, Lo JA, Maher K, He Y, Tang X, Lam J, Chen H, Li B, Fisher DE, Liu J, Wang X. (2023) Spatiotemporally resolved transcriptomics reveals the subcellular RNA kinetic landscape. Nat Methods [Epub ahead of print]. [article]