Autoimmune diseases are a group of diseases caused by abnormal immune responses to functional body parts. Single-cell RNA-sequencing (scRNA-seq) technology provides transcriptomic information at the single-cell resolution, thus offering a new way to study autoimmune diseases. Most single-cell RNA-seq studies, however, have often focused on one type of autoimmune disease.
scRNA-seq data was integrated from peripheral blood cells of five different autoimmune diseases (IgA nephropathy [IgAN], Kawasaki disease [KD], multiple sclerosis [MS], Sjogren’s syndrome [SS], and systemic lupus erythematosus [SLE]). Dimensionality clustering, cellular communication analysis, re-clustering analysis of monocytes, NK cell populations, differential gene expression analysis, and functional enrichment was performed for all immune cells in these data.
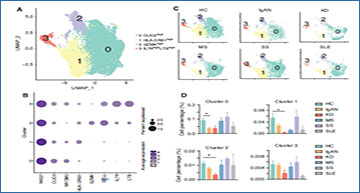
The scRNA-seq results of peripheral blood cells from five different autoimmune diseases (IgAN, KD, MS, SS, and SLE) were integrated. All samples contained 18 different immune cell subsets, although the cell cluster populations were different among the 5 diseases. Through intercellular communication network analysis, researchers determined that the signals of classical and non-classical monocytes were significantly enhanced in patients with IgAN and SLE. The signals of naïve B cells were increased in patients KD. Interestingly, the signals of NK and NK-T cells were enhanced in patients with SS, but reduced in patients with IgAN and SLE. Transcriptomic analysis of classical and non-classical monocyte subsets further revealed that pro-inflammatory cytokines and interferon-related genes, including CCL3, IL1B, ISG15, and IFI6, were specifically increased in patients with IgAN and SLE. Unlike monocytes, the number and NK marker genes were decreased in patients with IgAN and KD, but increased in patients with SS. Meanwhile, two NK-T cell subsets were exclusively found in SS.
scRNA-seq revealed the different phenotypes of NK cells in five autoimmune diseases

(A) UMAP illustrating NK cell subsets of all groups. (B) Dot plot of marker genes in each subset of NK cells. (C) UMAP illustrating the distribution of NK subsets in each group. (D) Histograms of the percentage of cells in the NK subsets in each group. *P < 0.05 vs. HCs. (E) Violin plots of the specific upregulated genes in patients with MS and SS, and downregulated genes in patients with IgAN and KD. (F) Feature plots of specific upregulated genes in patients with SS and downregulated genes in patients with IgAN.
In summary, based on an integration of the single-cell RNA-seq results, these researchers demonstrated changes in the immune cell landscape of five different autoimmune diseases with respect to immune cell subsets, populations, differentially-expressed genes, and the cell-to-cell communication network. The data provides new insight to further explore the heterogeneity and similarity among different autoimmune diseases.
Luo S, Wang L, Xiao Y, Cao C, Liu Q, Zhou Y. (2023) Single-Cell RNA-Sequencing Integration Analysis Revealed Immune Cell Heterogeneity in Five Human Autoimmune Diseases. BIO Integration [Epub ahead of print]. [abstract]