As the pandemic continues, millions of people in the U.S. are facing long COVID, a complex, poorly understood chronic illness with symptoms that can linger for months or even years after a patient gets COVID-19.
But a Duke researcher has identified the reason behind one of the chronic symptoms of long COVID — a perpetual loss of smell.
According to Bradley Goldstein, an ear, nose and throat specialist at the Duke Otolaryngology and Oral Surgery Clinic, the necessity for this study, which was published in Science Translational Medicine, arose as patients recovering from viral symptoms endured a prolonged loss of smell.
To uncover the mechanisms behind prolonged loss of smell, researchers analyzed biopsies of the tissue inside the nasal cavity lining of subjects with a loss of smell, as well as subjects with a normal sense of smell.
They did this using single cell RNA-sequencing, which Goldstein described as “a way to, at a cell by cell level, profile exactly what each cell is doing in terms of its gene expression and activity.”
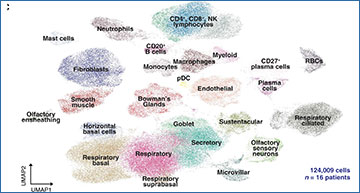
T cell infiltrates in nasal olfactory epithelial biopsies from PASC hyposmic patients
(A) Representative immunohistochemistry images of nasal biopsy tissue from normosmic non–COVID-19, normosmic post–COVID-19, or PASC hyposmic individuals. Tissue sections were immunostained for the TUJ1 neuronal marker, CD45 pan-immune cell marker, CD3 T cell marker, and CD68 myeloid cell marker. PASC hyposmic tissue showed dense CD45+ immune cell infiltration, including prominent CD3+ lymphocytic infiltration, which was absent in the normosmic groups; scattered CD68+ cells were present in all conditions. (B) Enlarged area (yellow box) from (A) shows CD3+ lymphocytes, with prominent infiltration into the olfactory epithelium (white arrows); dashed white line marks the basal lamina. Scale bar, 50 μm. (C) Additional nasal biopsies were processed for scRNA-seq to permit quantitative analyses. Uniform manifold approximation projection (UMAP) visualization of combined PASC hyposmic and control normosmic scRNA-seq datasets integrating 16 human nasal biopsies permitted robust cell cluster analysis and annotation. RBCs, red blood cells; pDC, plasmacytoid DCs.
According to Goldstein, the research team found that there was unresolved inflammation in the olfactory lining.
“Specifically, we’re seeing some types of immune cells that aren’t normally present in the controls … And we think that those immune cells, specifically T cells, and macrophages, are very likely causing some problems,” he said.
Goldstein said that when someone’s sense of smell is damaged, people realize how important smell is to their daily lives.
“We’re constantly getting information through our olfactory system about the world around us,” he said.
Not only does the olfactory system facilitate the sense of smell, but it also affects memory and emotion due to how olfactory input is wired to the brain, according to Goldstein.
“If all of a sudden, that entire sensory modality is just shut off, people really find that disconcerting,” he said.
But identifying the reason is just the first step.
“There’s really not a lot that we have to offer to help people recover their sense of smell right now. We certainly don’t have any specific drugs that have been designed to target exactly this problem and help correct it. So I think that that’s definitely an unmet need,” Goldstein said.
Because olfactory neurons are exposed in the nasal lining, these drugs can potentially be topical.
“That might allow us to overcome limitations … so you don’t necessarily have to take something systemically as a pill or a shot,” he added.
The next steps in this research include expanding the sample size and utilizing the information gleaned from the study to develop long-term treatments.
Source – Duke University
Finlay JB, Brann DH, Abi Hachem R, Jang DW, Oliva AD, Ko T, Gupta R, Wellford SA, Moseman EA, Jang SS, Yan CH, Matsunami H, Tsukahara T, Datta SR, Goldstein BJ. (2023) Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci Transl Med 14(676):eadd0484. [article]